Mnova Combos allow you to enjoy several Mnova plugins at the same time while you get important savings!


|
An advanced tool for 3D conformational and configurational analysis using experimental constraints from NMR spectra Mnova StereoFitter computes the probability of 3D structural configurations and/or conformations, based on various forms of NMR experimental data input. Currently, StereoFitter can accept four distinct types of input in order to calculate the best 3D structure candidate(s): NOEs, RDCs, Js, and chemical shifts. > GET A LICENSE |
|
StereoFitter

|
Computes the probability of 3D structural configurations and/or conformations Selects the simplest model for you Results are presented in order of best fit Various formats of external structure files are compatible: SDF; XYZ; MAE Chemical shifts can be computed within StereoFitter using DFT |
StereoFitter
What’s new in Mnova StereoFitter 1.1.3?The latest version 1.1.3 was released with Mnova 14.3 and these are the main highlights: Improved workflow in 3D correlations widget for NOE distances calculations: Synchronize the Assignments table with the 3D Correlations table Warnings are displayed when the tables are out of sync to allow the user to pick which table to overwrite the other Enhanced the ‘Run StereoFitter’ progress dialog: Show a chronological list of events that happen during the fitting process by pressing a new ‘More’ button on the dialog window Provided support for ReSpect: StereoFitter can now import files generated by ReSpect software Molecules that are topologically identical (same structure) that have a different numbering system when imported to StereoFitter will be renumbered to match the active molecule (usually the assigned 2D molecule)
|
 |
StereoFitter
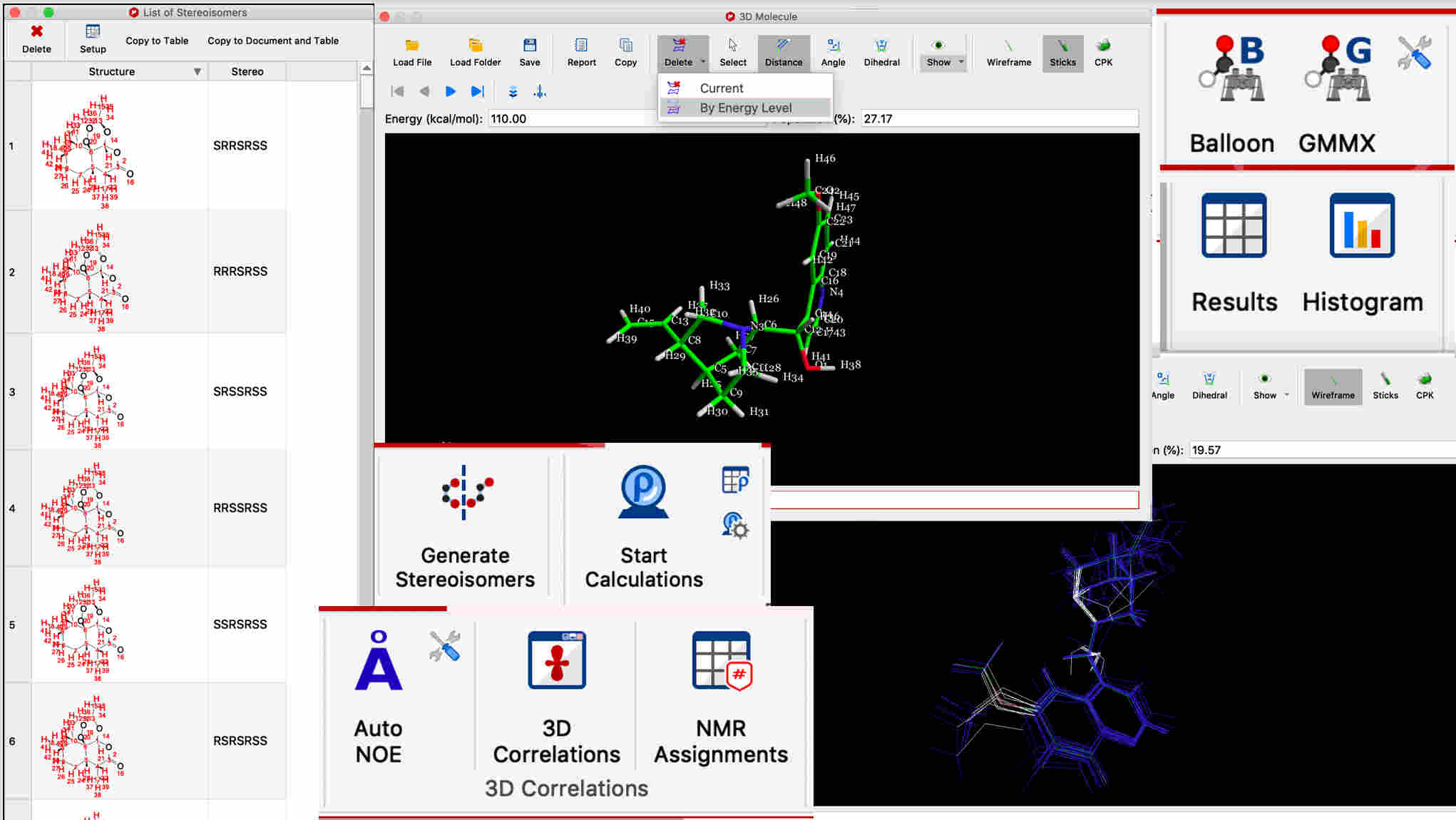
3D conformations can be calculated from the 2D structure
Sets of 3D conformations can be interactively viewed, aligned, overlayed, and filtered by energy level
Stereoisomers can be computed from any 2D structure with ambiguous stereo centers
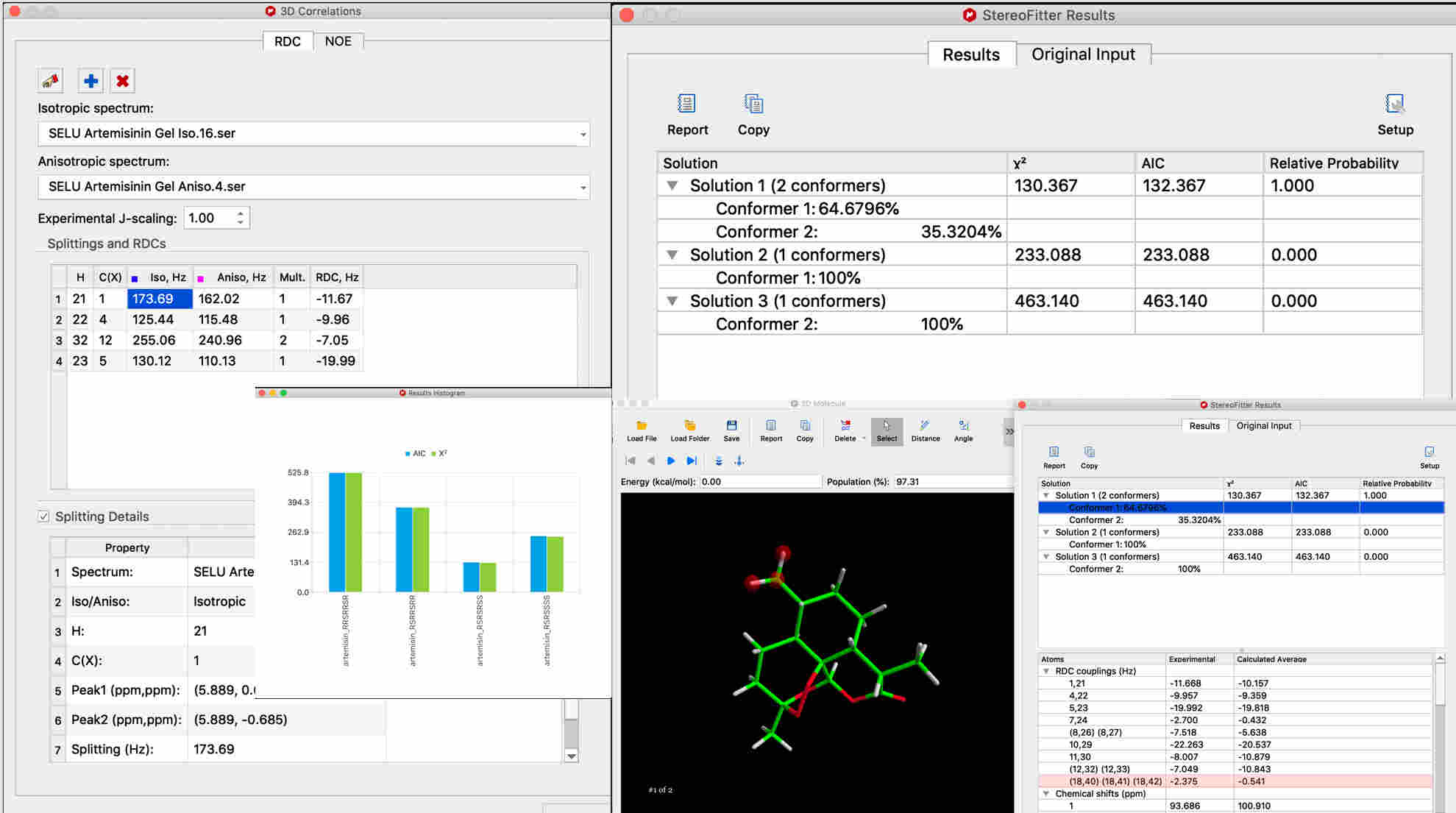
All types of measured constraints can be used in one 3D calculation for more accuracy and precision
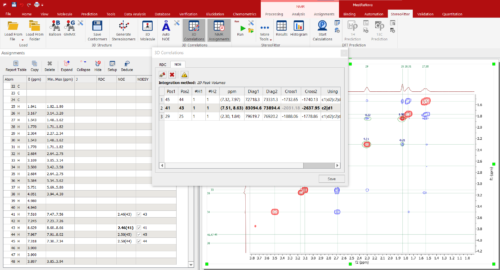
For NOEs, distances are easily calculated from measured cross-peak volumes with a single click.
 You can find an example in the manual (p.27) of Mnova StereoFitter using NOEs to determine 3D structure from a set of conformers.
You can find an example in the manual (p.27) of Mnova StereoFitter using NOEs to determine 3D structure from a set of conformers.
For RDCs, automatically calculate RDC values from isotropic and anisotropic spectra.
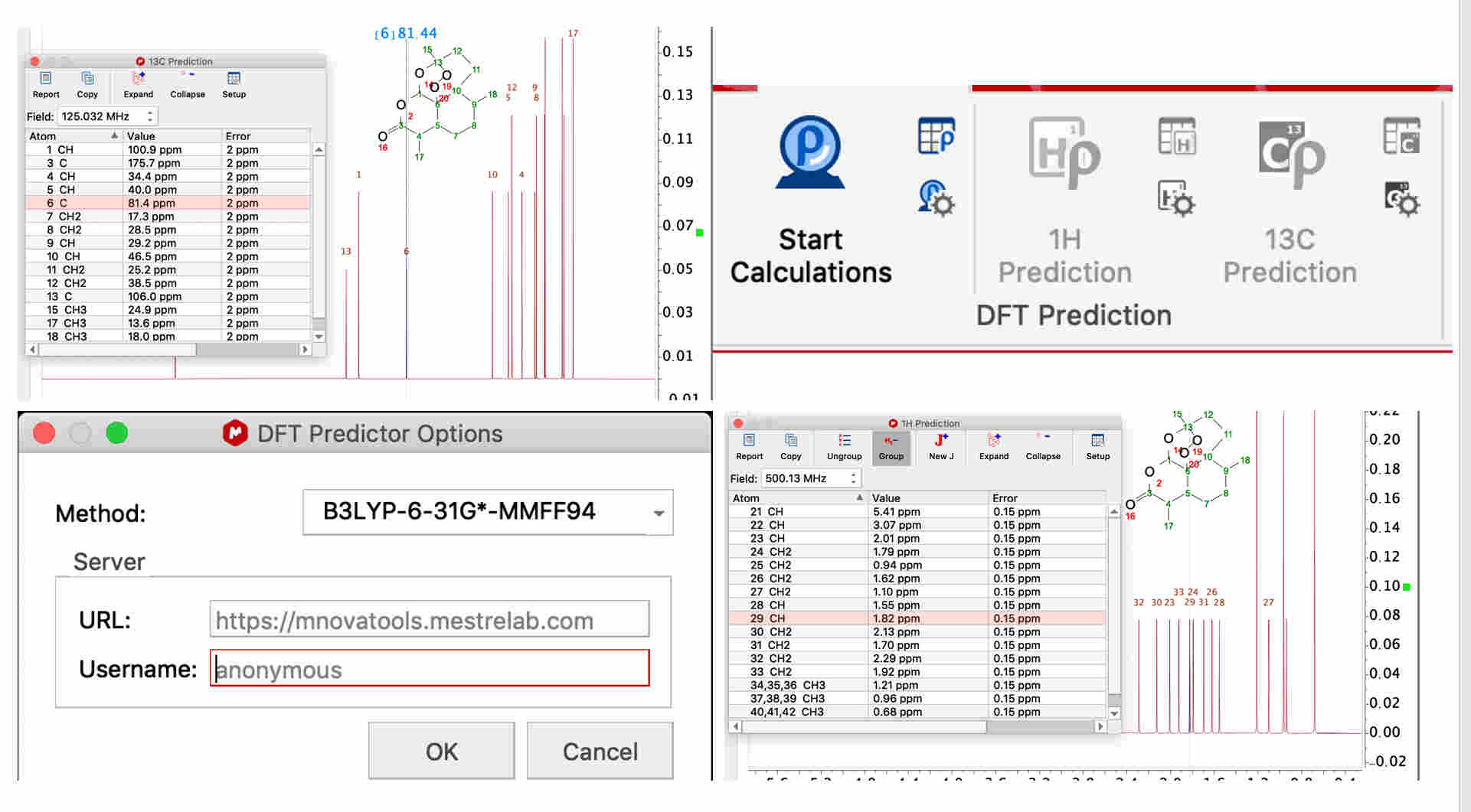
For chemical shift prediction, DFT calculations can be carried out within StereoFitter. A separate DFT Predictor license is required for running these calculations. The DFT Predictions section of the ribbon contains tools to predict 1H or 13C chemical shifts using results from a server-based DFT program.
StereoFitter Academic, Government & Industrial
|
|
StereoFitter
|
✈:No. 377, Nanjing Road, Shibei District, Qingdao, Shandong
☏:0532-83818797 / 18561885100
✉:changzhu_ ji@tlwb.com.cn shuochao_dai@tlwb.com.cn support@tlwb.com.cn
Learn more |
 |
